Meet The SNCE800 Series™
SNCE800 series is a family of ICs consisting of an Analog Front-end (AFE), Analog-to-Digital Converter (ADC), and Digital Signal Processor (DSP).
SNCE800 measures activity from the Brain (EEG), Heart (ECG), Muscles (EMG), and other bio-electrical signals from the body with unparalleled signal quality against low power consumption.
It features a real-time electrode impedance check, and an onboard shield driver and has ultra-high input impedance and low leakage current, making SNCE800 compatible with dry electrodes as well as gel-based electrodes.
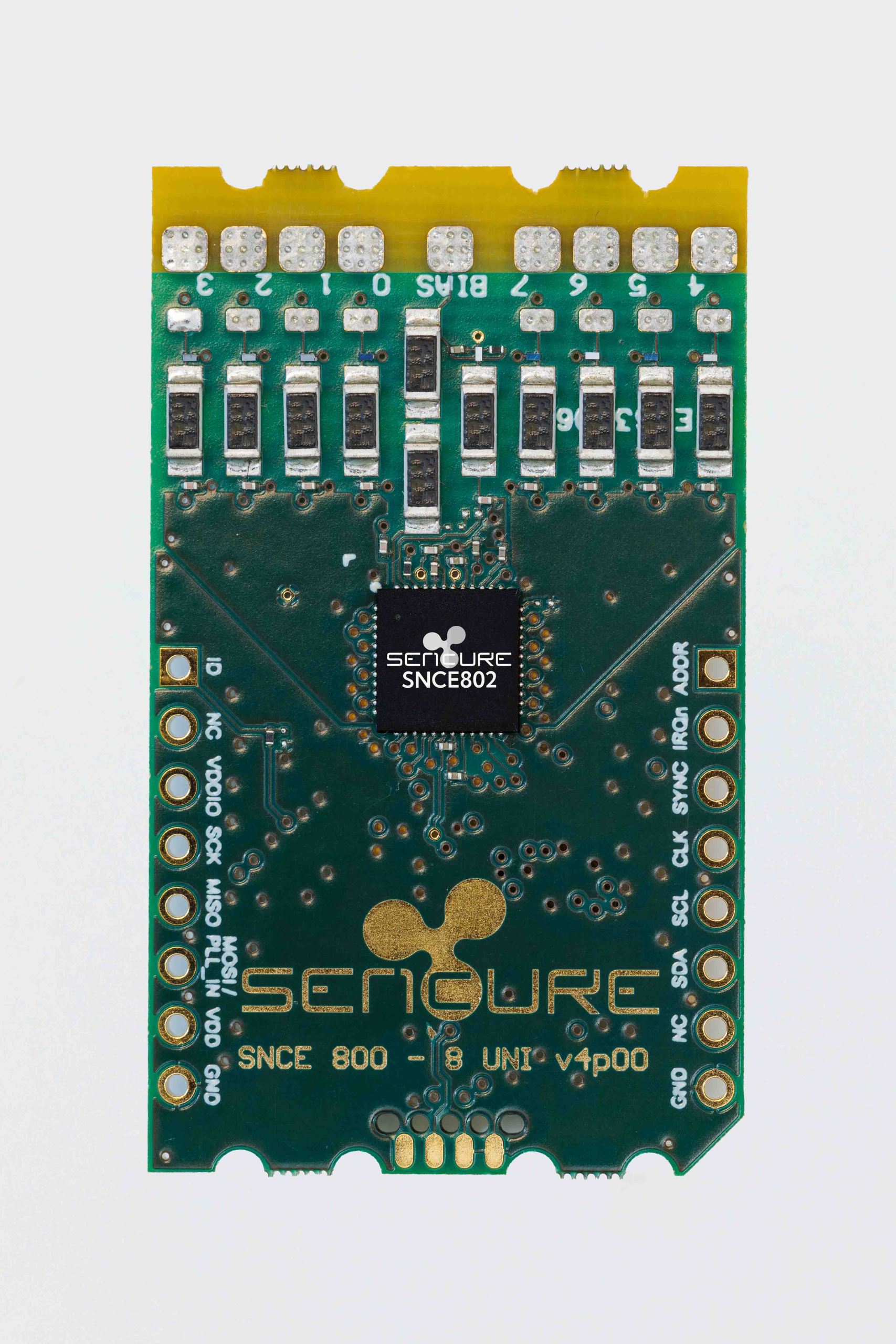
The SNCE800 series consists of an 8-channel (SNCE808), a 4-channel (SNCE804), and a 2-channel version (SNCE802).
Evaluation Kit
An evaluation kit for the SNCE800 series is available for a select number of customers. The evaluation kit features the SNCE800 with 9 connected patient connection leads (8 channels and 1 BIAS lead). It is soldered on a board with a MikroBUS™ interface allowing for interfacing to a variety of microcontrollers for data read-out. The evaluation kit comes with an extensive integration guide explaining everything that is needed to evaluate our chip.
The evaluation kit is only available after careful selection by Sencure. Please fill out the form below to book a meeting so we can explore potential collaboration at our booth!
Who are we?
Sencure is a start-up based in the Netherlands with a mission to enhance wearable devices in both consumer electronics and medical device industries. Measurement of bio-electrical signals can be challenging where signal quality and power consumption are usually very hard to combine in an optimal way.
Sencure has developed an AFE, ADC, and DSP in one, that will measure electrophysiological signals with unparalleled signal quality against minimal power consumption. This allows for much smaller and more advanced wearable devices.
Looking for Partnerships
Sencure is looking for partnerships with Medical Device Manufacturers, Contract Engineering Companies, and Companies developing Wearable Devices for Consumers that wish to bring their wearable devices to the next level. Together we can bring innovation to many different applications, whether that is for remote patient monitoring, diagnostic devices, or sleep and health tracking devices. SNCE800 can be a good solution for your product.
Sencure is looking for partnerships that embrace the value SNCE800 brings their product and for partners that are seeking viable alternatives to the existing solutions such as the ADS1299, ADS1298, or similar analog front-ends.
Contract Engineering Company
Our advanced technology provides cutting-edge solutions, while the reduced hardware complexity and seamless integration of our chips streamline the production process for contract engineers. This results in maximum flexibility to meet specific customer requirements and accelerates the time to market.
Benefit from our ready-to-use reference designs and documentation. Our SNCE800 series enables you to develop medical devices and wearables much more efficient.
Health Tracking Manufacturer
Medical Device Manufacturer
SNCE800 allows for smaller medical devices, better signals and easier compliance to 60601-1 Basic Safety regulations. SNCE800 comes with a ready-to-use reference design for medical devices that can be certified and comply with 60601-1 requirements.
Book a Time Slot
Experience how Sencure supports wearable innovation with cutting-edge technology and customized solutions. Learn about Evaluation Kits, explore design services, and discuss potential collaborations during CES2025. Our team at CES is ready for you! To make sure the right person is there to talk to you, book a timeslot in advance!

Get in touch with our experts
or call us: +31 (0)85 24 64 950
See more contact info below this page.